SigzenPHARMA is a purpose-built pharma ERP software solution developed by Sigzen Technologies for the pharmaceutical and Active Pharmaceutical Ingredient (API) manufacturing sector. Built on the powerful ERPNext and Frappe framework, this solution enables pharma businesses to seamlessly align with GMP, FDA, and 21 CFR Part 11 compliance requirements. Whether you’re an API manufacturer, bulk drug producer, or formulation-based plant, SigzenPHARMA provides the digital tools needed to optimize pharmaceutical operations, ensure batch traceability and drug traceability, and support regulatory audit readiness.
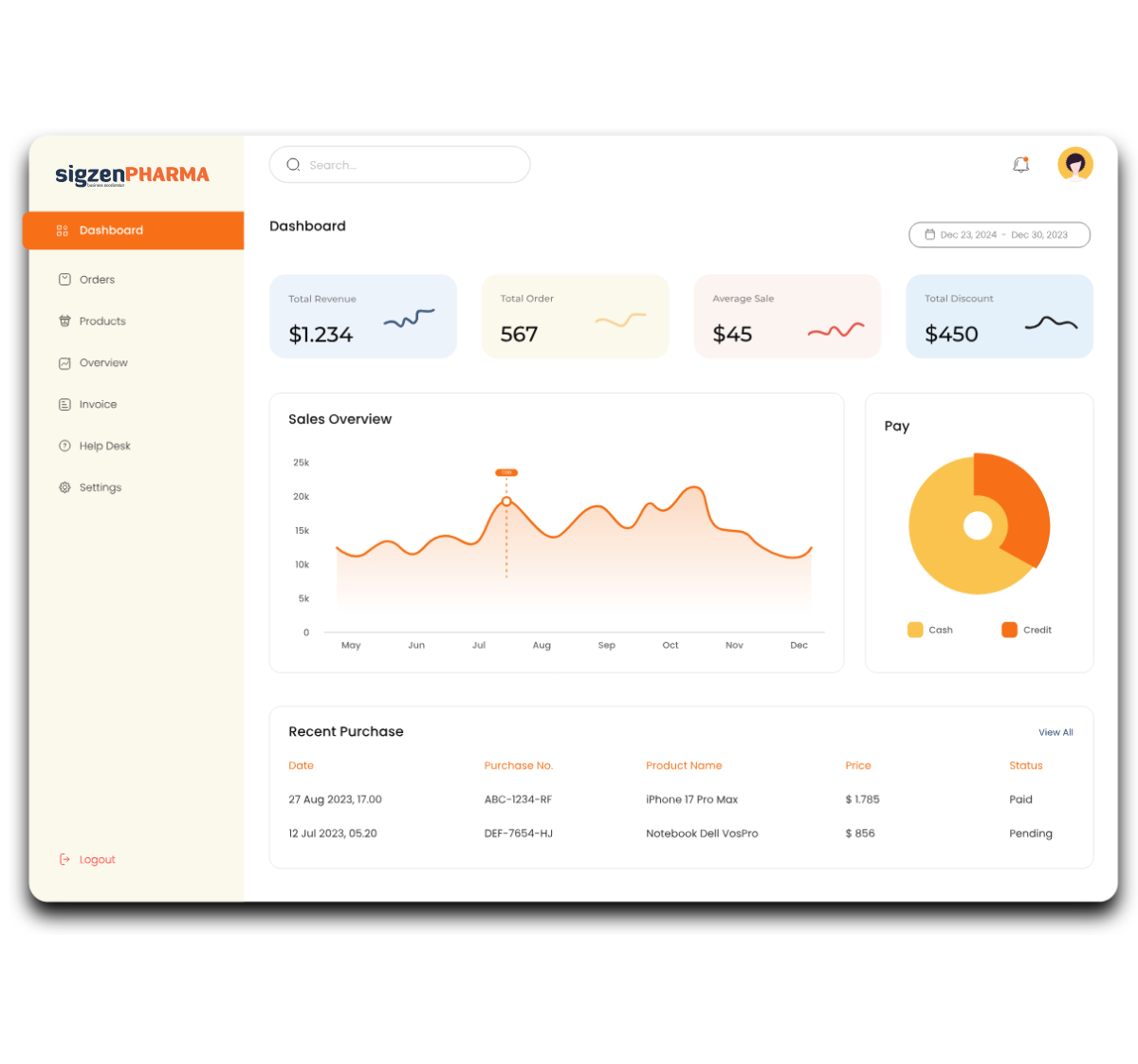
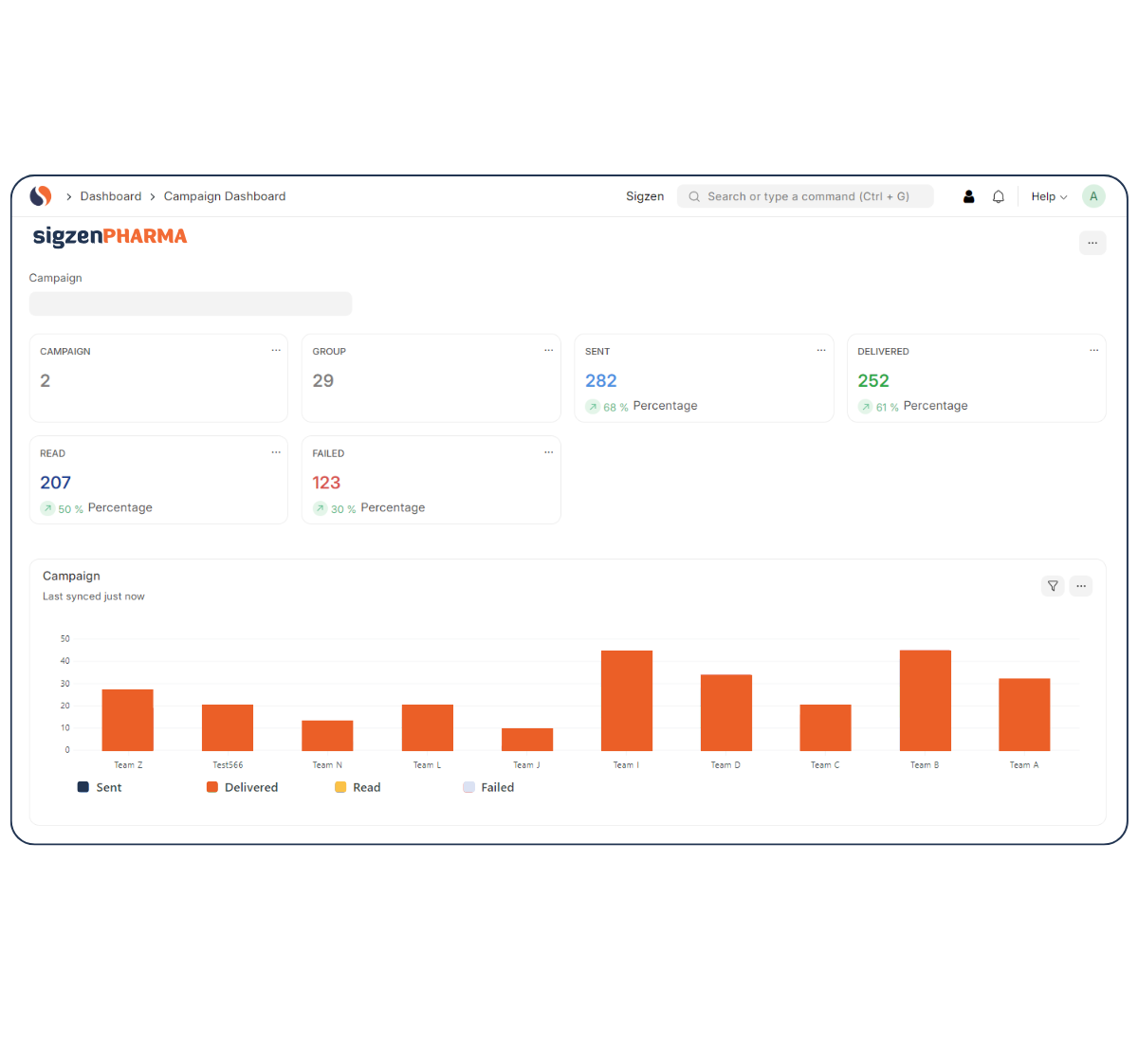
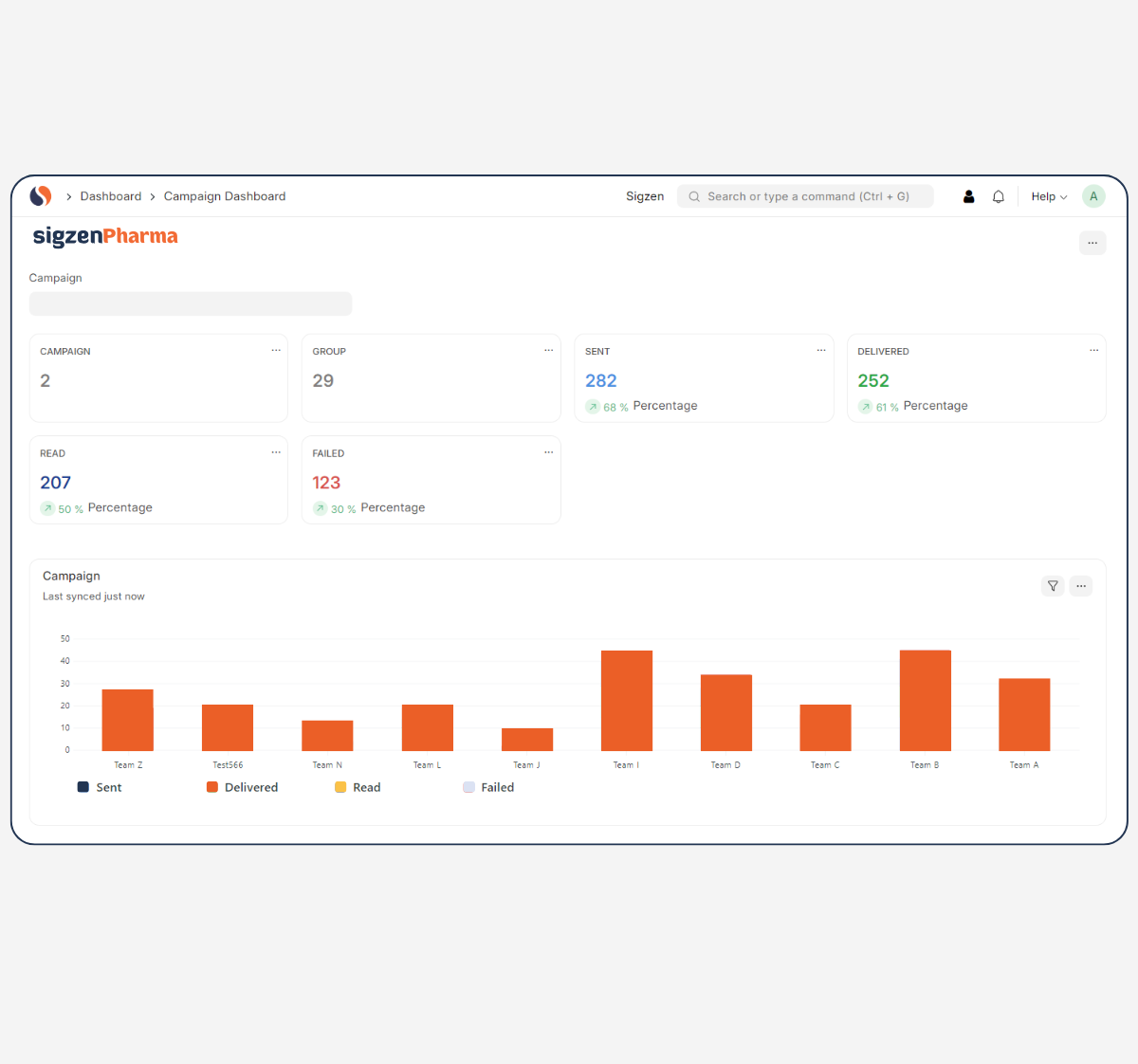
Designed with a deep understanding of regulated environments, SigzenPHARMA simplifies GMP-compliant documentation, improves data integrity, and automates the full production-to-quality lifecycle. With in-built support for batch manufacturing records, quality control protocols, validation, and real-time manufacturing analytics dashboards, this ERP is your partner for compliant growth in the pharmaceutical space.